Pharmaceutical
TeraView has successfully applied its proprietary terahertz imaging and spectroscopy technology to increase pharmaceutical product and process understanding as an effective part of the quality by design initiative.
Our systems utilise 3D terahertz pulsed imaging to nondestructively estimate critical quality attributes in pharmaceutical and medical products such as crystalline structure, thickness and chemical composition. TeraView Pharma Innovations team has demonstrated terahertz instruments produce 3D coating thickness maps for multiple coating layers and structural features models allowing better understanding and control of product scale up and manufacture.
The TeraView proprietary terahertz platform can be used to non-destructively:
- Develop robust product and processes for scale up to manufacture.
- Design and predict changes in drug release profiles.
- Troubleshoot product failures at all stages of development related to dissolution changes,
core integrity or other factors. - Monitor product attributes affecting stability.
Controlling Dissolution during Tablet Scale Up
Terahertz peak amplitude is an indicator of dissolution and a measurement of coating density. It is used to maintain and ensure in-specification performance during scale up of a sustained release product. Unlike coating weight gain, Terahertz peak amplitude considers the porosity of the coating.
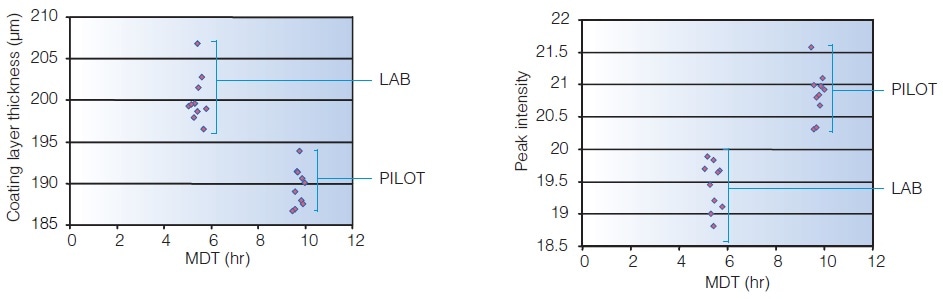
Predicting Dissolution through Tablet Imaging
Terahertz imaging reveals why tablets coated to release drug in the lower intestinal tract are dissolving in an erratic manner. For coating thickness, the mean dissolution time correlates to the terahertz measurements. A potentially quicker technique is offered by terahertz imaging for establishing variation in dissolution rates.
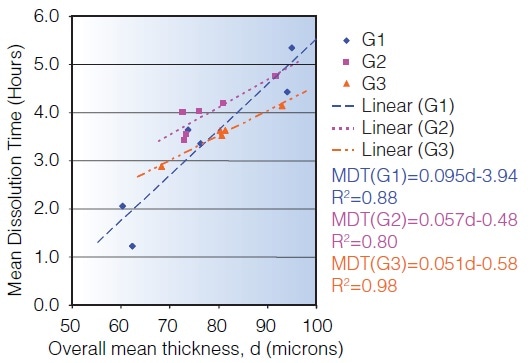
* Data courtesy of JA Spencer, FDA from a study published in the Journal of Pharmaceutical Sciences.
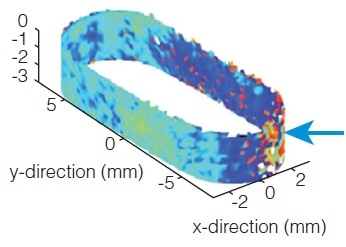
Controlling Process Transfer of a Multi-layer Tablet
A non-destructive measurement of the buried interfaces between two layers of a bi-layer product can be made using Terahertz imaging. The measurement allows the compression and formulation parameters to be optimized for better adhesion between the layers in the tablet core.
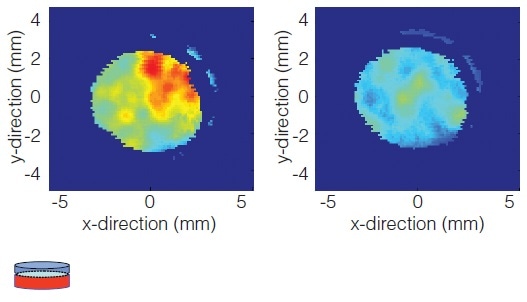
Effects of Film Coating Process on a Finished Drug Product
In this case, terahertz imaging monitors the impact of changing the solids level of a coating suspension from 15% to 25% to bring down the coating process time. The result shows a change in adhesion between the substrate and coating justifying additional investigation on stability.
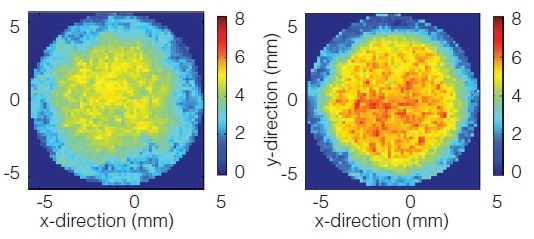
Defining the Compression Process Window
Terahertz imaging identifies compression process limits for the manufacture of a tablet prone to latent capping. Those compression parameters that cause cracking (yellow/red areas), deep in the tablet core, are exposed by virtual cross sections of the tablet. Consequently, the compression parameters can be more accurately defined to guarantee high quality of the finished product.
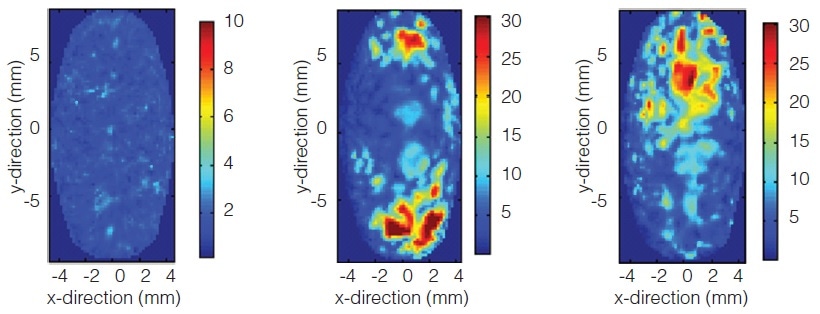
Investigating Failure in an Enteric Coated Product
Terahertz imaging non-destructively reveals poor sub-coat uniformity beneath an enteric coating that was not evident through the usual method of measuring weight gain. The resulting dissolution failure is due to interaction between the enteric coating and the tablet core.
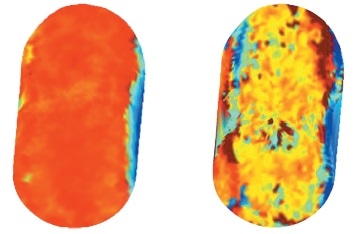
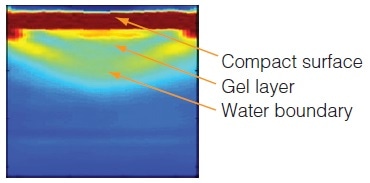
Determining Moisture Uptake and Gel Formation in Modified Release Tablets
Moisture uptake via coatings and in compacts made from several controlled release grades of hypromellose is effectively monitored by terahertz imaging. Images, captured within the first few minutes of water application, provide an insight to the hydration process and show how terahertz can be employed to investigate drug release rates and measure the thickness of gel layers as well as rate of diffusion.
Teraview Terahertz Pulsed Imaging Technology Adds Value
- No sample preparation and versatility to evaluate a wide range of solid dosage forms regardless of shape and size.
- Use non-destructive imaging to analyse effects of formulation and process changes on the structure and critical quality attributes of a dosage form.
- Build surface and depth data into dosage form knowledge. See details in the tablet such as agglomeration, fractures, interaction between cores and coatings, coating thickness and uniformity.
- Gain in-depth information from non-invasive 3D dosage mapping with full virtual cross-sectioning capability.
- Correlate terahertz information with other tests on the same tablet for successful root cause analysis.